Reduced Inflammation
Inflammation is a natural immune response that occurs when the body’s defense mechanisms are activated to protect against infections, injuries, or other harmful stimuli. The primary objective of inflammation is to remove the cause of the damage and initiate the healing process. Inflammation can be classified into two types: acute and chronic inflammation. Acute inflammation is a short-term response that typically lasts from a few hours to a few days, whereas chronic inflammation is a prolonged and persistent immune response lasting for months or even years.
Chronic inflammation can result in tissue damage and contribute to the development of various health conditions, such as heart disease, diabetes, obesity, and certain cancers. This low-grade inflammation can be triggered by factors such as poor diet, lack of exercise, stress, or exposure to environmental toxins. At the cellular level, chronic inflammation involves the sustained activation of immune cells, which release pro-inflammatory cytokines and other mediators that can cause damage to healthy tissues and disrupt normal cellular functioning.
The ketogenic diet has been shown to have potential anti-inflammatory effects. Let’s take a deep dive into the specific ways the ketogenic diet can reduce inflammation in the body.
Beta-Hydroxybutyrate
The primary goal of the ketogenic diet is to induce ketosis, where the body switches from using glucose for energy to using ketones derived from fats. Ketones, particularly beta-hydroxybutyrate (BHB), have been found to exert anti-inflammatory effects through various molecular mechanisms, which can contribute to the potential health benefits of the ketogenic diet.
Inhibition of the NLRP3 inflammasome
One of the primary ways BHB exerts its anti-inflammatory effects is by inhibiting the NLRP3 inflammasome, a multiprotein complex responsible for activating the inflammatory response. The NLRP3 inflammasome, when activated, leads to the release of pro-inflammatory cytokines, such as interleukin-1β (IL-1β) and interleukin-18 (IL-18). BHB has been shown to block the assembly of the NLRP3 inflammasome, thereby preventing the release of these pro-inflammatory cytokines and reducing inflammation.
Activation of the AMPK signaling pathway
BHB can activate AMP-activated protein kinase (AMPK), a key cellular energy sensor that plays a critical role in regulating metabolism and inflammation. Activation of AMPK has been linked to reduced inflammation through the suppression of nuclear factor-kappa B (NF-κB), a transcription factor that regulates the expression of various inflammatory genes. By activating AMPK, BHB can downregulate NF-κB signaling and decrease the production of pro-inflammatory cytokines.
Modulation of reactive oxygen species (ROS) production
BHB has been shown to reduce the production of reactive oxygen species (ROS), which are highly reactive molecules that can damage cellular components and promote inflammation. BHB can enhance mitochondrial efficiency and reduce the generation of ROS, which can contribute to its anti-inflammatory effects.
Upregulation of anti-inflammatory genes
BHB can also upregulate the expression of genes that have anti-inflammatory properties, such as those encoding for antioxidant enzymes and heat shock proteins. These proteins help protect cells from damage and maintain cellular homeostasis, which can contribute to the overall reduction of inflammation.
Reducing Blood Sugar Spikes
The reduction in carbohydrate intake on a ketogenic diet can also contribute to reduced inflammation. High-carbohydrate diets, especially those rich in refined sugars and processed foods, can promote inflammation by causing rapid spikes in blood glucose levels and insulin. In contrast, the low carbohydrate intake on a ketogenic diet leads to more stable blood glucose levels and lower insulin levels, which can help reduce inflammation.
Let’s discuss the main underlying mechanisms that cause elevated levels of inflammation.
Increased production of reactive oxygen species (ROS)
When blood glucose levels rise rapidly, the body’s cells need to process and utilize the excess glucose. This process can lead to an increased production of ROS, which are highly reactive molecules that can cause oxidative stress and damage cellular components. Oxidative stress can, in turn, activate pro-inflammatory signaling pathways, resulting in an inflammatory response.
Activation of pro-inflammatory pathways
High blood glucose levels can activate several pro-inflammatory pathways, such as the nuclear factor-kappa B (NF-κB) signaling pathway. NF-κB is a transcription factor that regulates the expression of various inflammatory genes, including those encoding for cytokines, chemokines, and adhesion molecules. Activation of NF-κB leads to the production of these pro-inflammatory mediators, which can cause inflammation and tissue damage.
Formation of advanced glycation end products (AGEs)
Rapid blood glucose spikes can lead to the formation of AGEs, which are the result of non-enzymatic glycation reactions between glucose and proteins, lipids, or nucleic acids. The accumulation of AGEs can cause cellular dysfunction and activate the receptor for advanced glycation end products (RAGE), which further triggers pro-inflammatory pathways, such as NF-κB signaling. This process ultimately results in the release of pro-inflammatory cytokines and the promotion of inflammation.
Endothelial dysfunction
High blood glucose levels can also cause endothelial dysfunction, which is characterized by impaired vasodilation and increased vascular permeability. Endothelial dysfunction contributes to inflammation by promoting the adhesion and infiltration of immune cells, such as monocytes and neutrophils, into the vessel wall. These immune cells release additional pro-inflammatory mediators, exacerbating the inflammatory response.
It is essential to remember that adopting a low-carbohydrate diet can significantly contribute to reducing inflammation in the body. By limiting carbohydrates, particularly refined and processed varieties, you can help prevent rapid blood glucose spikes that trigger inflammation.
Omega 3’s
Furthermore, the ketogenic diet encourages the consumption of healthy fats, such as those found in avocados, nuts, seeds, and olive oil. These fats contain anti-inflammatory compounds, such as omega-3 fatty acids, which have been shown to modulate immune responses and decrease the production of pro-inflammatory cytokines.
An optimal omega-3 to omega-6 fatty acid ratio is crucial for maintaining proper inflammatory responses in the body. These essential fatty acids play a critical role in regulating inflammation, cell signaling, and overall health. Let’s discuss the science behind how a balanced ratio of omega-3 and omega-6 fatty acids can reduce inflammation.
Biochemical Pathways
Omega-3 and omega-6 fatty acids serve as precursors for the production of eicosanoids, which are signaling molecules that have potent effects on inflammation and immune responses. Omega-6 fatty acids, particularly arachidonic acid (AA), are metabolized into pro-inflammatory eicosanoids such as prostaglandins, thromboxanes, and leukotrienes. In contrast, omega-3 fatty acids, including eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), give rise to anti-inflammatory eicosanoids and other lipid mediators, such as resolvins and protectins. An optimal omega-3 to omega-6 ratio helps maintain a balance between pro-inflammatory and anti-inflammatory eicosanoids, reducing the overall inflammatory response.
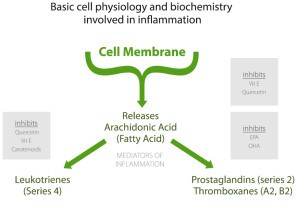
Competition for Enzymes
Both omega-3 and omega-6 fatty acids compete for the same enzymes, such as delta-6-desaturase, which are responsible for their metabolism. When the ratio of omega-3 to omega-6 fatty acids is balanced, the enzymes can effectively process both types of fatty acids, leading to a balanced production of pro-inflammatory and anti-inflammatory mediators. However, when the ratio is skewed toward a higher omega-6 intake, the enzymes become saturated with omega-6 fatty acids, reducing the conversion of omega-3 fatty acids into their anti-inflammatory derivatives. This imbalance can result in a predominantly pro-inflammatory environment.
Gene Expression
Omega-3 fatty acids can also influence the expression of genes involved in inflammation. For instance, they can suppress the activation of the nuclear factor-kappa B (NF-κB) signaling pathway, which is a key regulator of pro-inflammatory gene expression. By modulating the activity of transcription factors such as NF-κB, omega-3 fatty acids can help reduce the production of pro-inflammatory cytokines and chemokines, ultimately dampening the inflammatory response.
Cellular Membranes
The incorporation of omega-3 fatty acids into cell membranes can also affect cellular signaling and inflammation. Higher levels of omega-3 fatty acids in cell membranes can lead to an increased production of anti-inflammatory eicosanoids and lipid mediators while decreasing the availability of arachidonic acid for pro-inflammatory eicosanoid synthesis. Additionally, omega-3 fatty acids can modulate the fluidity and function of cell membranes, which can influence receptor-mediated signaling and cellular responses to inflammatory stimuli.
Quick Summary
Inflammation is a natural immune response to infections, injuries, or other harmful stimuli. It is designed to protect the body by removing harmful agents and initiating the healing process. However, when inflammation becomes chronic, it can lead to tissue damage and several health conditions like heart disease, diabetes, obesity, and certain cancers. Chronic inflammation is usually triggered by factors such as poor diet, lack of exercise, stress, and environmental toxins.
The ketogenic diet is known for its potential anti-inflammatory effects. It works primarily by inducing ketosis, where the body switches from using glucose for energy to using ketones, specifically beta-hydroxybutyrate (BHB). BHB inhibits the NLRP3 inflammasome, activates the AMPK signaling pathway, reduces the production of reactive oxygen species (ROS), and upregulates anti-inflammatory genes, thereby reducing inflammation.
The ketogenic diet also reduces inflammation by stabilizing blood glucose levels. Rapid blood glucose spikes, common in high-carbohydrate diets, increase the production of ROS, activate pro-inflammatory pathways, form advanced glycation end products (AGEs), and cause endothelial dysfunction, all leading to inflammation.
Moreover, the ketogenic diet encourages the consumption of healthy fats, including omega-3 fatty acids, known for their anti-inflammatory properties. Omega-3 and omega-6 fatty acids influence the production of eicosanoids, signaling molecules that affect inflammation and immune responses. A balanced ratio of these fatty acids is critical for maintaining a balanced inflammatory response. Omega-3 fatty acids also influence gene expression and cellular membranes, further contributing to anti-inflammatory effects.
In conclusion, the ketogenic diet’s potential to reduce inflammation lies in its ability to induce ketosis, stabilize blood sugar levels, and encourage a balanced intake of omega-3 and omega-6 fatty acids.


